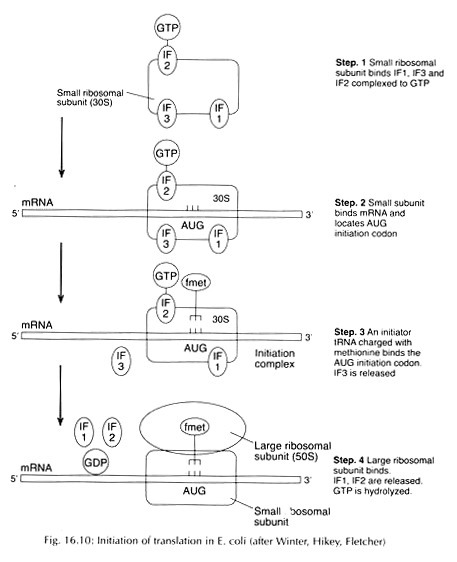
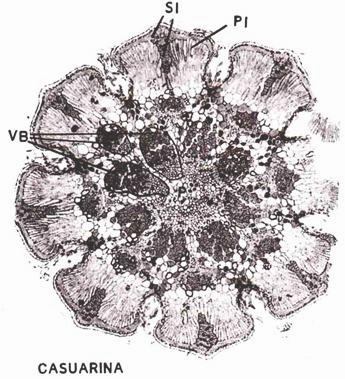
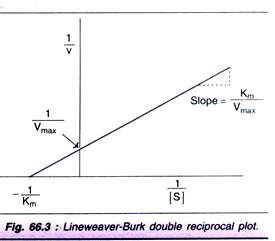
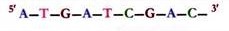ADVERTISEMENTS:
Water is the essential solvent of life and the main chemical constituent of all cells.
The essential role played by water can be attributed to its unique physical and chemical properties, which collectively act to protect living systems and are necessary to the structure and function of cells.
One of the unusual characteristics of water is that despite its low molecular weight, it is a liquid at most environmental temperature.
ADVERTISEMENTS:
Other molecules having similar molecular weights (e.g., ammonia, methane, and H2S) are gases.
In comparison with other solvents, water has high values for melting point, heat of fusion, boiling point, heat of vaporization, specific heat, and surface tension (see Table 3-4). Each of these properties serves to constrain major temperature fluctuations and to keep water in the liquid state.
Water absorbs more heat energy per gram for every degree rise in temperature than other common solvents and therefore acts to moderate temperature changes. (Indeed, the calorie is specifically defined as the amount of energy required to raise the temperature of one gram of water from 14.5° to 15.5°C.)
Likewise, to convert one gram of water from the liquid to the vapor (gas) state at its boiling point, an additional 2259 J (540 cal) must be absorbed. This high heat of vaporization, together with the high surface tension of liquid water, tends to keep water in the liquid state. At the other temperature extreme, large amounts of energy (335 J or 80 cal per gram) must be lost for water to be converted from the liquid to the solid state.
ADVERTISEMENTS:
Although water is often called the “universal solvent,” not all substances dissolve in water. However, water does dissolve most salts and other ionic compounds, as well as nonionic polar compounds such as sugars, alcohols, and other molecules that contain hydroxyl, aldehyde, and ketone groups. Many substances that contain both polar and nonpolar groups (such as soaps, fatty acids, and glycero-phosphatides) do not dissolve in water, but they do form micelles.
A micellar arrangement is not a true solution but is a suspension or dispersion. The behavior of soap molecules in water is a good and also common example of micelle formation. Soap molecules, formed by the saponification of fatty acids, consist of a long, nonpolar hydrocarbon chain terminating in a polar carboxyl group that is ionically bonded to a metal ion such as K+ or Na+.
When dispersed in water, the soap molecules aggregate to form spherical clusters, called micelles, in which the polar carboxyl groups of the soap molecules are arranged at the surface of the sphere, where they form weak bonds with the surrounding water, and the nonpolar hydrocarbon chains project inward (Fig. 3-1).
The special physical properties of water are founded in its molecular structure. In water, two hydrogen atoms are each covalently bonded to a single oxygen atom. The oxygen atom, being more electro- philic than the hydrogens, attracts the shared electrons more strongly and produces an electrical asymmetry in the molecule. As a result, the oxygen atom takes on less than a full negative charge, technically called a partial negative charge and denoted by δ–.
The hydrogens become partially positive (Fig. 3-2). Because the bond angle between the hydrogen atoms is 104.5°, each water molecule acts as a dipole even though it has no net charge. Because of their dipole character, water molecules tend to form bonds with each other.
The bonds linking neighboring water molecules are called hydrogen bonds and are formed by the attractions of the electronegative oxygen of one molecule for the electropositive hydrogens of two other molecules. Thus, by means of two covalent bonds and two hydrogen bonds, each oxygen atom may bond to as many as four hydrogens; the hydrogens form a tetrahedron about the oxygen (Fig. 3-3). The hydrogen bonding that occurs between water molecules in liquid water (Figs. 3-2 and 3-4) accounts in part for water’s high heat of vaporization and surface tension.
When the temperature of water is lowered, the concomitant decrease in kinetic energy allows the water to become denser until at 4°C maximum density occurs. A continued decrease in kinetic energy allows more extensive and less transient formation of hydrogen bonds among the water molecules. This promotes the development of a lattice structure (Fig. 3-4). As the freezing point of water is approached, more space develops between the molecules and the density decreases; thus, freezing water and ice rise to the surface of the liquid.
Ionic substances are readily dissolved in water because of the water molecules’ dipolar character. When salts (or other polar compounds) are dissolved in water, the orientation of the water molecules with respect to each other is disturbed. For example, sodium chloride (one of the most common salts found in cells and tissues) ionizes in water to form Na+ and CI–; these ions attract the water molecules and disturb the water lattice. Dissolved cations and anions convert the water lattice into spheres of hydration that encage the ion (Fig. 3-5).
Were it not for the formation of spheres of hydration about their ionic groups, many large molecules such as proteins would not be soluble in water. By encaging the ionized groups of proteins in layers of water, the groups are prevented from interacting with each other.
ADVERTISEMENTS:
As a general rule, ionized salts bind water more strongly than the polar groups of macromolecules, and this property is occasionally used to separate macromolecules from their solvent. For example, when salts are added to a protein solution, the salt ions form more extensive hydration spheres than the proteins. In effect, the redistribution of the water around the salt ions diminishes the amount of solvent available for the ionized groups of the proteins.
The ionized groups of the proteins interact with each other, and a protein precipitate is formed. This method for separating proteins from their solvent is called “salting out”.
Water molecules also undergo ionization; the capacity of water to form ions is another of its important biological properties. As we have already seen, in water each hydrogen atom is covalently bonded to one oxygen atom and forms a weak electrostatic bond with an oxygen atom of another water molecule (i.e., the water molecules are hydrogen bonded to one another). With measurable frequency, the covalent bond linking a hydrogen to oxygen is broken and the hydrogen establishes a closer association with the oxygen to which it was electrostatically bonded.
The water molecules have thus ionized, dissociating into a hydroxide anion (OH–) and a hydronium cation (H3O+). At standard temperature and pressure, one liter of water contains 1.0 x 10-7 moles of both OH– and H3O+. Both the hydronium ions and hydroxide ions form hydrogen bonds with other water molecules. The notation H3O+ is rarely used; H+ (called a hydrogen ion or proton) is used more frequently, even though free protons do not generally exist per se but are bonded to water to form hydronium ions.