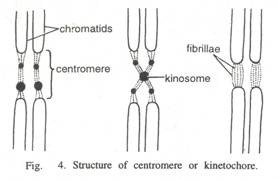
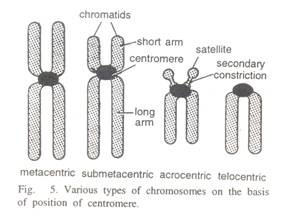
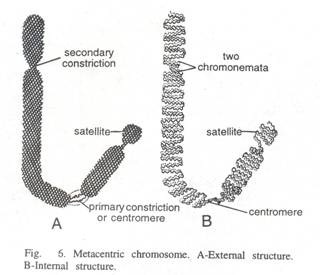
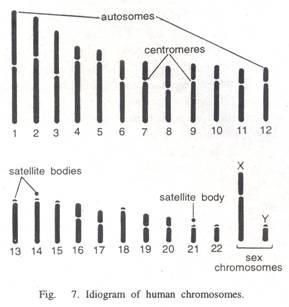
ADVERTISEMENTS:
The below mentioned article provides a practical guide to microscopes, drawing appliances and measurement.
Light Microscope:
The different components of a light microscope are shown in Fig. 1.1. The path of light through a microscope is shown in Fig. 1.2. The most important parts of a microscope are its lenses, of which there are two — objective and eye-piece (ocular), — and also the condenser which collects and focuses light on the object.
The light, after passing through the condenser, the object (in Plane I) and objective, will form a real and inverted image (in Plane II) if the eye-piece were removed. In presence of ocular F, the light rays are intercepted, forming the image (in Plane III).
ADVERTISEMENTS:
This real image is now examined with the eye lens E of the ocular and forming a virtual image (in Plane IV). The distance between the virtual image (Plane IV) and the eye point is called the projection distance, which is usually 250 mm.
The distance between the two lenses is known as the tube length. This is usually 160 mm in most microscopes. Total magnification is the product of the magnifying power of the objective and the magnifying power of the eye-piece.
It may thus appear that the magnification will go on increasing as more and more powerful lenses are used. In practice, however, this is not possible as magnification is limited by resolution or resolving power of the lenses.
Resolution and Numerical Aperture:
ADVERTISEMENTS:
It may be noted that if an object is observed under 10X ocular and 40X objective, i.e., at a magnification of X400, the image is distinct and has full clarity. However, if it is observed under 15X ocular and 40X
objective, i.e., at a magnification of X600, the image will be bigger but indistinct and rather hazy. This is exactly where the problem of resolution comes in.
The resolution (resolving power) of a microscope is based on the capacity of its lenses to distinguish details and to produce distinct images of objects that are very close together. In other words, the limit of resolution is determined by the ability of the lens system to collect light and the wavelength of light used.
The numerical aperture (N. A.) is a measure of the capacity of a lens to utilize a cone of light and is found out by the expression N.A. = n sin u, where n is the refractive index and u is half of the magnitude of the angle produced by the cone of light coming out of the object and entering the objective; i.e., ½ ![]() AOC =
AOC = ![]() AOB in Fig. 1.2.
AOB in Fig. 1.2.
Numerical aperture is written on the body of the lens. The N.A. of the low power objective is 0.25, that of the high power objective is 0.65, and that of the oil immersion lens is 1.3 or 1.4. The resolving power is determined by the formula
Resolution (r) = λ/2NA
where X (lambda) represents the wavelength of light. Visible or white light has a wavelength range of 3,900 Å (blue-violet) to 7,600 Å (red), 1 µ (micron) is equal to 10,000 Å (Angstrom units). So, with white light, having an average wavelength of 5,500 Å (0.55 µ), the limit of resolution is about 0.25 µ.
For example, the brightest part of the spectrum has a wavelength of 5.300 Å. An objective with a numerical aperture of 1.00 will resolve two lines separated by a distance of 5,300/ (2 × 1.00) = 2,650 Å (95,000 lines to an inch, i.e. 2.54 cm).
The higher the numerical aperture, the better the resolution. But there are limitations here, because the glass lenses used in a light microscope prohibit the passage of short wavelengths of light. Approximately, the smallest detectable detail in a light microscope is equal to about half the wavelength of light is used for observation.
Oil Immersion Lens:
ADVERTISEMENTS:
A light microscope usually has three objective lenses; 10X, 12.5X or 15X (low power objective), 40X or 45X (High power objective) and 90X or 100X (oil immersion objective). When low or high power objectives are used the field of observation appears quite bright and the object is well-clarified.
But when the oil lens is used, the field of observation appears poorly illuminated and the object is also ill-clarified. This is because when the light enters from a denser medium (cover glass) into a lighter medium (air lying between cover glass and lower end of objective) it becomes divergent.
So only a fraction of the light that comes out of the object can enter the objective and, when the oil lens is used, it becomes quite inadequate to produce a clear image.
To overcome this difficulty an oil (immersion oil or cedar wood oil) is placed on the cover glass and the oil lens is dipped in it to eliminate the intervening air — so that all the light rays which pass through the object can enter the objective and the image becomes well-clarified. The slide and the oil lens are cleaned with xylol after use.
ADVERTISEMENTS:
An object is first brought at the centre of the field of observation and then the high power is brought in. The object is now sharply focused with slight adjustment of the fine adjustment screw.
Such a microscope is called per-focal. Sometimes it is found that an object brought at the center of the field of observation under low power recedes to the periphery or goes out of the field. Such a microscope is said to be not properly centered.
Aberrations:
No man-made lens system is perfect. All of them exhibit some form of optical aberration — chromatic or colour aberration is the foremost amongst them. Lenses are essentially of prism system which have the property of breaking down white light into the different component colours.
They are, therefore, made in such a manner so as to bring back the different colours of light into white light again. An objective of this type is called an achromatic or apochromatic objective. A greater degree of perfection is obtained in an apochromatic.
ADVERTISEMENTS:
An eye-piece corrected for chromatic aberration is called a compensating, hyper-plane or Huygenium eye-piece. Maximum perfection is obtained with a compensating eye-piece. The other types of aberration encountered are spherical aberration, distortion, curvature of field, coma, astigmatism and lateral colour (Fig. 1.3).
Condensers:
A good microscope is fitted with a condenser which is placed just below the stage and so is called sub-stage condenser. A good condenser sends light through the object under an angle sufficiently large to fill the aperture of the back lens of the objective. It is essential for observation with the oil immersion lens and is also preferable while using the high power dry objective.
It is also corrected for the various optical aberrations. The Abbe condenser and the variable focus condenser have 1.25 N. A. The best condenser is the achromatic condenser with N.A. of 1.4. In some microscopes an iris diaphragm is used instead of a condenser to control the cone of light entering the microscope (Fig. 1.3).
Dark Field Condenser:
ADVERTISEMENTS:
Ordinarily, in a microscope light is allowed to pass through an object and this is called microscopy by transmitted light or bright field microscopy. Many transparent objects are not easily visible in a bright field, such as cilia. In such cases visibility can be improved by using a dark field which allows only the light scattered or reflected by the object reach the objective.
This is done by inserting a dark field stop below the condenser, so that a hollow cone of light reaches the object. This method of illuminating an object, where it appears self- luminous against a dark field, is known as dark field illumination.
Simple or Dissecting Microscope:
This is a simpler version of the compound light microscope having a single lens (eye-piece or ocular) and a glass stage (Fig. 1.4). This is used for dissecting minute objects under microscope. Sometimes two metallic hand-rests are fitted to the sides of the stage for convenience in dissection.
Precautions:
1. Always clean the lenses with lens paper, clean linen or old and soft cloth before and after use.
2. Never focus under high power objective without a cover glass placed on the object.
ADVERTISEMENTS:
3. Never try to focus the object under high power objective directly.
4. Always focus under low power and then bring in the high power.
Phase Contrast Microscope:
Cells and their components are usually colourless, except some of the plastids. Because of this and their high water content, cells cannot be observed under light microscope without proper staining.
Most of the stains are toxic to living cells as they are dissolved in organic solvents, such as alcohol. So, the cells which are observed under light microscope are, actually, dead cells. Living cells, however, can be observed directly under phase contrast microscope.
A cell is rather heterogeneous in content and some of the components are thicker than the others. Moreover, the cell and its components have different refractive indices. A difference in either refractive index or thickness between two components produces a difference in phase.
The phase contrast microscope transforms the phase difference into brightness. Thus the transparent cell is observed in shades of grey which depend on the thickness and refractive index of the surrounding medium or background and of the different cellular components.
ADVERTISEMENTS:
The principle of the phase contrast microscope, therefore, is to convert small phase differences into differences in contrast that can be detected by the human eye. An annular (ring-like) diaphragm is placed in the condenser and an annular phase plate in the objective (Fig. 1.5).
The annular diaphragm illuminates the object with a narrow cone of light. Due to the presence of the annular phase plate, some of this light passes directly through the object and some light is diffracted laterally and an image of strong contrast is produced. The phase effect is the product of interference between the direct image in the centre of the objective and the diffracted lateral image.
Interference Microscope:
The interference microscope works on the same principle as the phase contrast microscope but it has some advantages. In the phase-contrast microscope there is a halo around each object in view. This can be eliminated in the interference microscope.
With the interference microscope it is easy to vary contrast and to select the most suitable contrast for an object. Colour effects can also be obtained and quantitative determinations, such as the dry weight of an object, can also be made. The interference microscope is, therefore, an improved version of the phase contrast microscope.
Polarizing Microscope:
The polarizing microscope is quite similar to the interference microscope. It utilizes the property of birefringence for observation. This method is useful for indirect analysis of the ultra-structures of the cells because the property of birefringence depends on structural properties smaller than the wavelength of light.
Ultraviolet Microscope:
In microscopes, one of the basic principles is that the shorter the wavelength of light used the greater the resolving power of the microscope. Glass lenses are used in light microscope which cannot transmit wavelengths below 4,000 Å.
If, instead of glass, fused quartz lenses are used, wavelengths of 1,400 Å or even lower can be utilized. Slides and cover-glasses are also made of quartz. Thus the range of ultraviolet light applicable to microscopy is about 2,000 to 4,000 Å.
The ultraviolet microscope is useful for qualitative — and sometimes quantitative — analysis of certain cytoplasmic organelles. For example, the absorption peaks of nucleoproteins lie within the range 2,600 to 2,700 Å and so it is possible to observe nucleoprotein containing materials, i.e. chromosomes, in unstained living cells.
The image produced is usually projected on a photographic plate. By means of spectrophotometer, the amounts of nucleoproteins in the cells can be determined by noting the peaks of ultraviolet absorption.
Fluorescence Microscope:
Ultraviolet light is also the source of illumination for the fluorescence microscope. When certain types of chemical substances are illuminated with ultraviolet light, excitations are produced in the molecules of the substance — causing them to emit light in the visible range. This is known as fluorescence. Substances like chlorophyll, riboflavin etc. can fluoresce readily.
This is called auto-fluorescence. Chlorophyll fluoresces red and riboflavin yellow-orange. Other substances such as DNA, RNA, proteins can be induced to fluoresce by the addition of specific dyes such as acridine orange (vital stain). This is called secondary fluorescence. These reactions in the living cell can be observed with the fluorescence microscope.
Usually ultraviolet light of 3,300 to 4,000 Å is used in the fluorescence microscope. The light emitted by the object has a different wavelength. Thus the object can be clearly differentiated. The ordinary light microscope can be used for fluorescence studies by relatively simple changes (Fig. 1.6).
A special filter capable of transmitting only the ultraviolet rays is placed near the source of light (a carbon arc or a mercury vapour lamp). Another filter — capable of absorbing the ultraviolet rays which are not absorbed by the object, and transmitting the visible light produced by fluorescence — is placed within the eye-piece.
X-ray Microscope:
As resolution depends upon the expression r = λ/2NA considerable improvement in resolution can be obtained with radiations of extremely short wavelengths (i.e., by reducing the value of X) such as X-rays. By using X-rays in the range of 1 to 10 Å, and sections 1 μ or less in thickness, molecular structures can be recorded on photographic films.
Electromagnetic lenses or reflecting curved mirrors focus the X-ray beams. Quantitative determinations of dry matter as also analysis of crystal structure can be made. There is one limitation in X-ray microscopy, i.e., only preserved materials can be studied.
More refined X-ray microscopic studies are based on the diffraction of X-rays when they encounter very small objects. This method was used by M. H. F. Wilkins to determine the structure of DNA for which he was awarded the Nobel Prize for Medicine along-with J. D. Watson and F. H. C. Crick in 1962.
Electron Microscope:
Principle:
The electron microscope offers the maximum resolution and so the highest magnification. It utilizes electrons of short wavelength (about 0.05 Å) as the means of illumination instead of visible light (5,500 Å). The smallest cells have a diameter of 0.1 µ with components which are obviously much smaller. Even in large cells, many of the components are less than 0.1 µ.
Such objects cannot be resolved under the light microscope. The electron microscope — using very short wavelength and so having a much reduced value of λ — is suitable for observation of such ‘ultramicroscopic’ structures. The components of a cell have different masses, each of which causes a differential scattering of the electron beam.
The contrast between an object and its surrounding medium, or between different objects, is produced by this differential scattering of the electrons by the atoms of the different objects — which permits the resolution of objects with diameters as low as 1 mµ or 10 Å, and, sometimes, even less. This represents resolving power 250 times that of the light microscope.
Components:
The body of the electron microscope (first developed in 1931) is quite complex and requires special training to handle it. However, the main components used in producing the image are, in principle, more or less the same as in a light microscope (Fig. 1.7).
The instrument is completely enclosed in vacuum, since electrons travel long distances only in vacuum. The source of the electrons is a cathode filament which emits a narrow beam of electrons. This beam is collected and focused on the specimen by an electromagnetic condenser lens.
The electrons then pass through the specimen and are collected again by an electromagnetic objective lens, which produces a magnified image. Further magnification is produced by an electromagnetic projector lens. It projects the image on to a fluorescent viewing screen or a photographic plate. The image cannot be viewed directly by the eye as in a light microscope, since the human eye is not electron-sensitive.
Focusing of the image can be adjusted by varying the flow of current in the projector lens. Both the lenses contribute almost equally to the magnification. A magnification of 160,000 times — and sometimes even more (90 million times by STEM — Scanning Transition Electron Microscope) — is possible with the electron microscope. The similarity of components of light and electron microscopes is presented in Fig. 1.8.
Section Cutting:
The preparation of cells or cell components for electron microscopic study is a very critical and laborious process and requires specialized techniques. A thick specimen will scatter electrons more than once and so cause poor resolution. The thinner the section, the better the resolution. In practice, however, there are limits to the thinness of the sections.
It is now possible to have sections as thin as 100 A without causing any physical or chemical damage to the cell components. The material must be dry. Proper fixation of the material is essential. This can be done in various ways. Osmium tetroxide (OsO4) and formaldehyde (HCHO) are two of the most commonly used fixatives (Fig. 1.9). Fixation by freeze-drying in vacuum is another method.
After fixation, the material is embedded in a resin such as araldite, or in a plastic material, such as methacrylate, and sections are cut on a special type of microtome with diamond or glass knives. A section is then placed on a very thin membrane (instead of a glass slide) preferably not thicker than 150 Å.
The membrane should be strong enough not to tear apart and should produce very little electron scattering. The membranes commonly used are made from collodion (nitrocellulose) or form-bar (polyvinyl formaldehyde). The membrane is supported by a metal grid of steel or copper.
Types of Electron Microscope:
There are two types of electron microscope — Transmission Electron Microscope (TEM) and Scanning Electron Microscope (SEM). In the first type the entire object is subjected to a beam of electrons and those electrons which pass through the object form the image on the screen.
In the scanning type the object is scanned with a small beam of electrons. The electrons which emit from the object are collected to form the image on a cathode ray tube.
Cytochemical Assay by the Use of Microscope:
Cytochemical techniques may be used to identify specific chemical components, especially enzymes, in cells, by direct microscopic observations of tissue sections in situ. The technique is based on the colorimetric detection of the components along with microscopic visualization. Some specific examples of cytochemical techniques are exemplified in the Table 1.1.
Micrometry Image Analysis and Video Microscopy:
These techniques permit measurement of length, area, depth, shape, surface density and other topographical features that may then be correlated with biochemical parameters. Micrometry, in simple terms, may be carried out using the eye-piece of a compound microscope fitted with an eye-piece graticule to measure.
Subsequently, the advent of microcomputers has greatly extended manipulation of numerical data for microscopic investigations. For example, digitizer pads allow operators to trace electronically with a pen the outline of images either projected directly on to the pad, or on photographs attached to the pad.
Modern image analyzers use closed-circuit television cameras to project the image on to a photocathode tube to produce a digitised picture. Digitised image are then stored in the computer memory or on disc for later processing and analysis.
Video microscopy can improve microscopic images in three ways. These comprise video enhancement microscope (VEM), which increases low contrast images electronically and video intensification microscopy (VIM), which amplifies low light images, e.g. from bioluminescence or a fluorescence and digital image processing. The basic equipment needed for video microscopy is shown in Fig. 1.11.
Video microscopy has great advantages for biochemical investigations because it allows not only image enhancement and improved resolution of low contrast images of living material, but also quantitative measurement of specific molecules to be followed over a specific time.
Drawing Appliances:
For an exact drawing of the image a camera Lucida, a drawing prism or a drawing head (Fig. 1.11) can be used. All the three instruments have the same working principle. The drawing prism is most commonly used. It has a prism which can reflect the image downwards at 45° angle.
The object is properly focused under the microscope, using the high power or oil lens — as is suitable. The eye-piece is removed and the hollow ring of the drawing prism is slid into the draw tube of the microscope. The eye-piece is replaced, the prism is brought over the eye-piece and is adjusted in such a way that it remains horizontal and just touches the eye-piece.
The hollow ring is now tightened with the metallic screw so that the prism does not move. The left eye is now placed over the eye-piece and the drawing paper on the table near the right hand side of the microscope. The viewer can see the image on the paper. A sharply pointed pencil held with the right hand is held over the paper.
Now both the image and the pencil can be simultaneously seen and an exact drawing is made. To have a distinct image it is necessary to adjust the light. The microscope light can be regulated by adjusting the level of the condenser and by adjusting its aperture. Light falling on the drawing paper can be regulated by placing an opaque body in front of it.
A microscope with a built-in light system is quite handy for this type of camera-Lucida drawing. A drawing head is more handy. It is simply placed over the eye-piece and the drawing is made. The camera Lucida is the oldest of the three models. It has a mirror fitted at the end of a handle which can be manually adjusted to reflect the image.
Magnification of the Camera Lucida Drawing:
A camera Lucida drawing becomes more magnified than the image seen directly by the eye because of angular deviation. As already stated, the camera Lucida image is reflected downward at 45° angle and this deviation further magnifies the image.
To find out the exact magnification, the slide is removed and a stage micrometer is placed under the microscope using the same combination of lenses. A stage micrometer has a scale where one mm is divided into 100 equal segments, so that one stage division is equal to 10 µ.
The stage micrometer is focused at the center of the field of observation and a few divisions are drawn with the help of the drawing prism. To get a more accurate result, the stage micrometer should be focused at the centre, at the left side and at the right side of the field of observation and three drawings should be made, because magnification varies according to the position of the stage micrometer.
It is greatest at the left end lowest at the right end of the field of observation. These divisions are now measured by a mm scale and the mean magnification of a stage micrometer division is found out by simple arithmetic.
Illustration:
1 stage micrometer division is magnified to 5 mm
i.e., 10 µ is magnified to 5 × 1,000 = 5,000 µ
1 µ is magnified 5,000/10 = 500 µ
ADVERTISEMENTS:
Magnification is 500 times.
Measurements:
For measuring the actual size of a cell or a microscopic plant organ a stage micrometer and an ocular scale or micrometer are necessary. The first step is standardization of the ocular micrometer.
The ocular scale is placed within the eye-piece and the stage micrometer on the stage. The two scales are so adjusted that they lie one above the other in a parallel manner. A number of readings are now taken indicating how many ocular divisions coincide with how many stage divisions.
The mean is then found out and calculations are made in the following manner:
Suppose:
10 ocular divisions coincide with 25 stage divisions
1 ocular division = 2.5 stage divisions
= 2.5 × 10 µ (1 stage div. = 10 µ)
= 25µ
Now the stage micrometer is removed and the object is placed there. The object is measured by the ocular scale and the actual size is calculated in terms of micron according to the standardize value of the ocular scale.
Example:
Suppose the mean length and breadth of a cell are 4.2 and 2.5 ocular divisions.
The length of a cell = 4.2 × 25 µ = 105 µ
And the breadth of a cell = 2.5 × 25 µ = 62.5 µ.
The size of a cell is 105 µ × 62.5 µ