ADVERTISEMENTS:
The following points highlight the top five classifications of isomerism. The classifications are: 1. D and L 2. α- and β-anomers 3. Epimers 4. Pyranose and Furanose Ring Structures 5. Aldose-ketose Isomerism.
Isomerism: Classification # 1. D and L:
The designation of an isomer as D- or L- form is determined by its spatial configuration to the parent compound. When the OH group around the carbon atom adjacent to the terminal primary alcohol carbon (carbon atom 5 in glucose) is on the right, the sugar is a member of the D series.
When it is on the left, it is a member of the L series. The majority of the monosaccharides occurring in mammalian metabolism are of the D configuration.
ADVERTISEMENTS:
Optical Isomer:
When a beam of polarized light is passed through a solution exhibiting optical activity, it will be rotated to the right or to the left in accordance with the type of compound, i.e. the optical isomer, which is present. A compound which causes rotation of polarized light to the right is said to be dextrorotatory and a plus (+) sign is used to designate the fact.
Rotation of the beam to the left (levorotatory action) is designated by a minus (-) sign.
When equal amounts of dextrorotatory and levorotatory isomers are present, the resulting mixture has no optical activity since the activities of each isomer cancel the other. Such a mixture is said to be racemic or a DL mixture. Stereoisomerism and optical isomerism are independent properties.
ADVERTISEMENTS:
Thus a compound might be designated as D (-) or L (+). Synthetically produced compounds are necessarily racemic. The separation of optically active isomers from a racemic mixture is called resolution i.e. the racemic mixture is said to be “resolved” into the optically active components.
Isomerism: Classification # 2. α- and β-anomers:
The cyclic structure of glucose is retained in solution, but isomerism takes place about position 1. This is accomplished by optical rotation (mutarotation) by which the positions of -H and -OH groups are changed around carbon.
a. Mutarotation:
Intra conversion of α & β-glucose in solution with change of optical activity, called mutarotation.
Mutarotation is explained by the α and β form of glucose requires the presence of asymmetric carbon c1.
In mutarotation hemi acetal ring is opened and reformed with change of position of -H and -OH.
Example 1:
When D-glucose is dissolved in water its specific rotation is 111°. When ammonia is passed, the value is 52.5°.
ADVERTISEMENTS:
b. If it is recrystallized from boiling pyridine, the rotation is 19° and finally 52.5°.
Isomerism: Classification # 3. Epimers:
Isomers formed as a result of interchange of the -OH and -H on carbon atoms 2, 3 and 4 of glucose are known as epimers. Biologically, the most important epimers of glucose are mannose and galactose formed by epimerization at carbons 2 and 4, respectively. In the body, epimerization takes place by the enzyme epimerase.
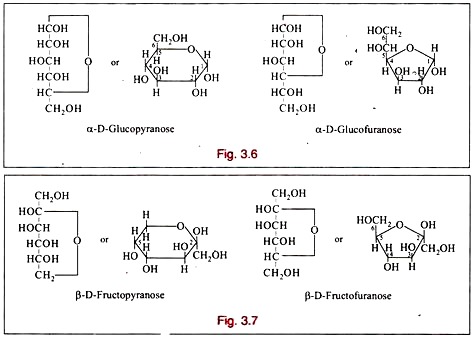
Isomerism: Classification # 4. Pyranose and Furanose Ring Structures:
Haworth, in 1929, proposed a scheme in which all sugars forming six-membered rings are called pyranoses from their relation to pyrane and those forming five-membered rings furanoses after furane.
The amylene oxide form of glucose would be called glucopyranose and the butylene oxide form glucofuranose.
If a sugar is referred to by its ordinary name only, the pyranose form is to be inferred. The pyranose and furanose forms of some sugars are set out below in straight chain fonnulae as well as in ring formulae.
Isomerism: Classification # 5. Aldose-ketose Isomerism:
ADVERTISEMENTS:
Fructose has the same molecular formula as glucose but differs in its structural formula since carbon 2 is a part of a > Co group, which makes fructose a ketose rather than an aldose. Generally, there is a free -H on carbon 1, the sugar is an aldose, but if a –CH2OH group is substituted, the sugar is a ketose (Fig. 3.8).