ADVERTISEMENTS:
In this article we will discuss about Gene Control and Transcriptional Control:- 1. Transcriptional Control in Yeast 2. Mechanism of Action of Activator and Repressor in Eukaryotes 3. Regulation of Gene Expression with Repressors 4. Some Examples of Gene Regulation in Eukaryotes 5. Translational Control at the Initiation Level 6. Gene Regulation at the Level of RNA Processing and Others.
Contents:
- Transcriptional Control in Yeast
- Mechanism of Action of Activator and Repressor in Eukaryotes
- Regulation of Gene Expression with Repressors
- Some Examples of Gene Regulation in Eukaryotes
- Translational Control at the Initiation Level
- Gene Regulation at the Level of RNA Processing
- Role of Chromatin Structure in Gene Regulation
- Transcription Signals for Gene Control in Eukaryotic Cells
1. Transcriptional Control in Yeast:
ADVERTISEMENTS:
Due to the smaller genome size and easy to perform genetical analysis in yeast, considerable work on transcriptional control has been done in this organism. Most of the mechanisms of transcriptional control in yeast have similarity with that of eukaryotic organisms. This has been further proved in getting same response of yeast transcriptional control sequences in mammalian cells (Figs. 16.8 and 16.9).
The transcriptional control sequences of yeast has 3 control elements:
ADVERTISEMENTS:
i. The TATA box,
ii. Initiation site and
iii. The upstream activation site (UAS).
UAS element has the same properties of the enhancer sequences of higher eukaryotes.
UAS element or site activates gene specific regulation, by binding the product of the regulator to the UAS site. For example, Galactose binds to the UAS site to confer transcriptional activation of genes encoding galactose metabolizing enzymes. There are various types of proteins from HAP1, HAP 2 and HAP 3 genes which activate transcription of a number of genes like CYC 1 gene encoding iso-l-cytochrome C.
There are some factors which can suppress transcription in yeast which is known as silencers (trans-acting proteins). Silencers have similarity with enhancers of eukaryotes in a number of characteristics. One such example is RAPI. But this RAPI protein may act as activator and repressor depending on the binding site.
2. Mechanism of Action of Activator and Repressor in Eukaryotes:
It is known that at a certain stage of development, each cell uses certain genes to direct production of other molecules and it is said that these are on, and those not expressed are off. Hence the expression of these genes is regulated.
This expression of genes is regulated not only during developmental stages but also during the lifetime of the differentiated cell. For example, cancer cells start division when normal cells do not. In cancer cells, certain genes are on when they should remain off.
ADVERTISEMENTS:
The first step in gene expression is the transcription of RNA from DNA. Transcription starts with the binding of RNA polymerase and other proteins to some specific DNA sequence (promoter) which then activates the gene (Fig. 16.10). The activity of RNA polymerase can be controlled by some regulatory proteins that bind to some specific sites of DNA.
The regulatory gene is flanked on both sides by the regulatory protein binding sites. One gene may have more than one regulatory gene with different sequences to which regulatory proteins bind. When the regulatory protein binds to correct sequence, transcription of gene starts with the help of RNA polymerase and other factors.
Regulatory sites may be present within genes also (Fig. 16.11). Protein- protein interaction takes place with the help of looping. The interaction takes place between proteins binding to separated sites on DNA with the intervening DNA forming a loop for doing the interaction.
In other words, the regulatory proteins bind to the specific sequence on DNA and can regulate transcription by touching proteins binding to adjacent sites or with certain other proteins. Eukaryotic genes are generally transcribed at low levels unless some activators are present.
Repressor proteins also regulate gene expression in eukaryotes by blocking the actions of activators. The action of eukaryotic activator is similar to that of repressors. In most of the eukaryotes, activator binding site is separated from the gene and so the intervening DNA must form a loop to make an interaction (Fig. 16.12).
The protein that binds with the activating region is called the target protein. About 50 mammalian proteins have been identified as activators— they activate the gene after matching with the proper binding site.
ADVERTISEMENTS:
The activating regions of DNA can vary in their strengths, i.e., one is more efficient than other when both are placed at an equal distance from the gene. This means that different activating regions bind with the target with different affinities.
In Fig. 16.13, stronger activating region is designated with four teeth and the weaker region is with two. When the regulatory site is present at great distances from the gene, it will activate gene if the activating region has stronger affinities. Again, a gene having more binding sites for an activator will be transcribed to a higher level if the activator has weak affinities.
3. Regulation of Gene Expression with Repressors:
ADVERTISEMENTS:
There are two types of repressors in eukaryotes which can regulate activation of genes. The first type is similar to a repressor which acts by steric occlusion. It binds with the binding site of activator in DNA tightly so that the actual activator cannot bind and thus repress by competition (Fig. 16.14).
The second type of repressor does not regulate through competition. These molecules have a DNA binding region and a repressing region which is analogous to the activating regions (Fig. 16.15). This second type of repressor thus nullifies the action of activators.
It is to be noted that the concentration of DNA is much higher than the concentration of the regulatory protein in eukaryotes. To overcome this problem single protein must have multiple binding sites or two different proteins may bind to the same site.
This phenomenon is called cooperatively or co-operative binding which plays an important role in eukaryotes than in prokaryotes. For example, protein X may bind to sites x and x’ and this protein X can interact with protein a or protein b which bind to adjacent sites (Fig. 16.16).
ADVERTISEMENTS:
Again, the level transcription can be changed or switched by the interaction of some non-identical proteins. For example, there are some proteins, say protein A—which bind to DNA without affecting any transcription [Fig. 16.17(a)].
The interaction of another protein B with A acts as an activator increasing the level of transcription [Fig. 16.17(b)]. Again, at a certain stage of development, another protein, C, binds with A replacing B, transcription is switched-off [Fig. 16.17(c)].
Thus C acts as a repressor here. This example shows that regulators from non-identical components can be used to minimize the regulatory molecules. In eukaryotes, extracellular signal, particularly hormone, has an important role in regulating gene expression.
In mammalian cells, certain genes show expression of one allele of the homologous pair. Sometimes the paternal inherited trait is expressed and, for others, the maternally inherited copy is expressed. This phenomenon of regulation in mammalian cells is called Imprinting. Again, methylation of DNA sequences may be involved in the gene regulation of eukaryotes.
ADVERTISEMENTS:
It has been well-established that eukaryotic DNA is wrapped in nucleosomes which also acts as a barrier to the transcriptional machinery. Although some activators act or bind to DNA while it is wrapped in nucleosome, most of the activators bind after histone dissociation in the nucleosome structure.
4. Some Examples of Gene Regulation in Eukaryotes:
(a) Mouse Aioumin Gene:
This gene is most active in liver cells. 200 base pairs upstream of this gene, having TATA sequence, contains five binding sites for activators. There is another 300 base pairs segments located about 10,000 bp upstream— this is necessary for the complete expression of the gene.
This distal site is known as enhancer which shows variations in effect when moved to varying distance from the gene. There are also three extra enhancer regions located about 30,000 downstream of the gene (Fig. 16.18).
(b) Gal Genes of Yeast:
The products of Gal genes are necessary for the utilisation of galactose as a carbohydrate source in yeast. When yeasts are grown in a medium without galactose, the Gal genfes remain silent, but these genes are expressed when galactose is present in the medium.
Two of the Gal genes Gal 1 and Gal 10 are shown in Fig. 16.19. Between these genes there is a segment of 118 bp with activating sequence known as upstream activating sequence (UASG). If the deletion of UASG occurs, Gal genes remain inactive.
Again, if the UASG is positioned within 750 base pairs of the gene, activation of gene starts with the decrease in activity as the distance from the gene is increased. In yeast, the product of Gal 4 genes has been identified as enhancer in the expression of Gal gene. 
5. Translational Control at the Initiation Level:
Several features of the 5′ non-coding regions of eukaryotic mRNAs are of importance for the translational control at the initiation level. These regions are 5′ cap structure [m7 G(5′)N; where N is of any nucleotide] and the 5′ non- coding region of mRNA. This 5′ cap structure is unique in the mRNA of eukaryotes except in organelles. The methylation occurs in 5′ end of mRNA which is greater in higher eukaryotes. The simplest cap structure (m7G-pppN) is found in lower eukaryotes like yeast which is known as “cap-O”.
Another important structure of eukaryotic mRNAs is the noncoding regions of 50-100 nucleotides in length which are devoid of AUG codons. In some organisms, longer non-coding regions are also found which have an important role in cellular growth and development.
The longest non-coding regions of 1,100 nucleotides is found in the Antennapedia locus of Drosophila melanogaster. Most of these regions contain AUG codons followed by termination codon. Another unique feature of this region is the presence of optional exon which helps in the post-transcriptional control. For example, half of the human and hamster transcripts for 3-hydroxy-3 methyl-glut-aryl-coenzyme-A synthase (HMG-CoA synthase) contain an optimal exon in the 5′-non-coding region. These non-coding regions can regulate the translational process in various organisms.
These are:
1. 5′ non-coding region of heat shock mRNA can initiate heat resistant translation.
2. Cap-independent translation in Polio- virus RNA by these regions.
3. Iron-mediated translational control in human-fenith mRNA by 5′ non-coding regions.
4. Control of amino acid synthesis by 5′ non- coding region of GCN4 mRNA.
6. Gene Regulation at the Level of RNA Processing:
The initial product of gene transcription is the heterogeneous array of RNA molecules (Hn- RNAs) whose sedimentation coefficients range from 20S to 100S. These RNA transcripts then undergo extensive processing during its conversion to messenger RNA. The existence of Gene control during RNA processing has been first noted in animal viruses, such as Adenovirus and SV40.
The primary transcripts can be spliced in different ways to produce several mRNAs. The same primary transcript of the Gene coding for a A2 crystalline lens protein can code for two polypeptide chains. Of them, one polypeptide chain contains 22 extra amino acid which is encoded by an exon, This extra segment is spliced out during mRNA processing in forming the alternative form of the protein (Protein XZ in Fig. 16.20).
This type of gene regulation is sometimes induced by mutations. Abnormal structure of globin protein—causing the disease Thalassaemia—is due to mutations near the ends of the intervening sequences. This leads to an abnormal splicing pattern producing the defective structure of haemoglobin.
This is one way of gene regulation in which any alteration in the pattern of splicing may make different types of messenger RNAs from the same transcript.
7. Role of Chromatin Structure in Gene Regulation:
There is a correlation between changes in chromatin structures and gene expression. It was noted long back that certain regions of eukaryotic chromosomes remain tightly condensed throughout the cell cycle. These condensed regions do not take part in transcription and the uncoiled regions take part in the transcription. The former is known as heterochromatin and the latter is euchromatin.
Heterochromatin is again divided into Constitutive heterochromatin and Facultative heterochromatin. Constitutive heterochromatin contains some DNA sequences which are never transcribed, such as satellite DNA of the centromere. Facultative heterochromatin contains DNA sequences that are capable of transcription. But these DNAs may be inactive in certain cell types as part of the developmental process.
The example of facultative heterochromatin is very clear in mammalian sex chromosomes. Murray Barr and others showed (1950) that the cells of female mammals have a small, darkly staining bodies of chromatin which is not found in cells of males.
This structure is known as Barr body, which shows that one X chromosome only. As this inactivation-occurs at random, some mature cells will derive from a cell lineage in which one X chromosome is converted into heterochromatin.
This inactivation of one X chromosome in females has been first shown by Lyon. So, all females contain one active X chromosome is inactive and other cells may derive from cells in which another X chromosome is inactive. Lyon also showed that genes for coat colours are present on two X chromosomes.
When the coat colours are present on two X chromosomes, the females may show mottled coats. For example, Tortoise-shell cat shows coat colours of yellow and black. This character is restricted to females only. This can be justified by stating that the expression of yellow colour is present on one X chromosome and the black colour is on another X chromosome.
Biochemical analysis of protein products from these genes also support the above idea. For example, electrophoretic analysis of one such product—Glucose-6-phosphate dehydrogenase—has proved this idea. The two forms of this enzyme has been obtained from the extracts of the human cell culture of two populations.
From the isolated single cells, clones were obtained which show only one type of band in each case but at two different regions (Fig. 16.21). Hence the original culture must have two types of cells showing the expression of genes present on two X chromosomes.
But there are other changes in chromatin structure which lead to the selective activation and inactivation of specific genes. Harold Weintraub and others showed that actively transcribed genes are more sensitive to DNAase I digestion than inactive genes.
DNAase I has an unique character of digesting DNA when it is still attached with nucleosomes. As for example, active globin gene of red blood cell chromatin is more sensitive to DNAase I digestion than the globin gene of brain cell chromatin where the globin gene remains inactive.
Again, it has been observed that sensitivity to DNAase I digestion does not depend on the process of gene transcription but it can explain the alterations in nucleosomal configuration of active or potentially active genes.
There are some sequences close to the transcriptionally active sites which can be digested with a trace amount of DNAase I. This region is known as DNAase-I hypersensitive site. This hypersensitive site may have some role in the binding of either RNA polymerase or regulatory proteins involved in transcription.
Several biochemical differences have been noted in the nucleosomes of active and inactive genes. But no difference in the nucleosomal organisation of active and inactive genes has been observed in the electron microscopic level.
The treatment of chromatin of active genes with DNAase I releases the acetylated form of H3 and H4, indicating thereby that acetylated histones are present in the chromatin of active genes. The presence of one type of polypeptide known as ubiquitin to histone 2A has been found to have some role in histone modification in both prokaryotic and eukaryotic cells.
The association of ubiquitin to the nucleosome structure leads to histone degradation giving a scope in the availability of the DNA for transcription. The role of another group of protein in altering the nucleosome configuration has been observed which is known as HMG (high mobility group) proteins.
Of these proteins, HMG 14 and HMG 17 are responsible for increasing DNAase I sensitivity upon active genes. Further gene regulation can be maintained by conferring changes in DNA structure such as gene amplification, gene deletion, DNA methylation, DNA rearrangements and changes in DNA conformation etc.
(a) Gene Amplification:
The rate of transcription of gene transcript depends on the presence of the number’ of copies of genes present in the cell. In case of salivary gland cells of Drosophila, thousands of copies of chromosomal DNA are present in polytene chromosomes giving rise to the synthesis of large quantities of RNA needed by these cells.
During the development of egg cells, multiple copies of the DNA sequence for ribosomal RNA are synthesised for the production of large number of ribosomes needed by these cells.
In amphibians, thousand-fold amplification of ribosomal genes occur during the developmental stages of egg cells. Again, insect eggs during development showed the selective replication of genes coding for chorion proteins when the proteins are required in large amount. This is another clear example of gene amplification. But these genes are located in the chromosomal DNA showing a specified area of polyteny.
Although the gene amplification of a certain gene occurs at a particular stage of development, there are some genes coding for different types of histones. These genes have multiple number of histone gene copies to cope with the rapid histone synthesis needed for packing the newly synthesised DNA into chromatin fibres.
(b) Gene Deletion:
In contrast to gene amplification, sometimes genes are deleted when their products are not needed. The good example of gene deletion is the red blood cell of mammals. These cells reject their nuclei after the synthesis of appropriate amount of hemoglobin.
In crustacean group of organisms, such as copepods, the heterochromatic regions of chromosomes axe deleted from all cells except the germ cells during early stage of development.
(c) DNA Methylation:
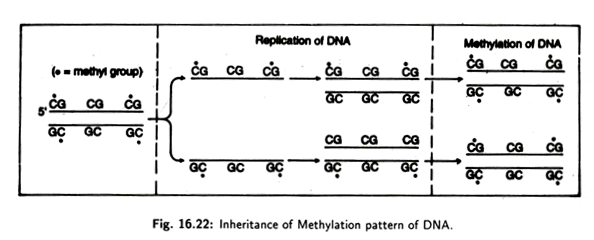
Most organisms contain a modified base known as 5-methyl cytosine which is produced by the transfer of a methyl group from S-adenosylmethionine to cytosine residues of the DNA chain with the help of an enzyme.
These methylated cytosine’s are located adjacent to a guanine residue forming a -CG- sequence. The enzyme methylases shows its activity at methylating -CG sequences which are base paired to -GC sequences where C is already methylated.
It has also been found that once the complementary pair has been methylated, this methylated condition will be inherited to the next generation during the process of replication. Thus, the DNA methylation patterns can be inherited from generation to generation (Fig. 16.22).
Some correlation between DNA methylation and gene activity has been found in many organisms. As for example, cytosine residues near the 5′ and of globin genes are methylated in cells where hemoglobin is not needed, but in red blood cells these cytosine’s are not methylated.
Again some correlation has been found between gene activity and decreased levels of DNA methylation. The role of methylation in gene activation may be shown in an experiment using a drug 5-azacytidine. This compound is a base analogue to cytosine which cannot be methylated as it contains a nitrogen atom in place of carbon atom where methylation occurs.
Hence, during replication, incorporation of 5-azacytidine into DNA shows an under- methylated state in the next generation even after the removal of the drug. 5-azacytidine treated cells showed either loss or decreased activation of genes.
Sometimes treated cells show different new types of gene activation. Even some hetero-chromatically inactivated X- chromosomes show gene activation in treated cells. But the role of DNA methylation in gene activation has been evidenced in all organisms, so no generalisations of its role can be made. Hence DNA methylation might be one of the different factors controlling gene expression.
(d) DNA Rearrangements:
This type of DNA alteration has some influence on gene activity because inherited alterations in base sequences exert some effects on gene activity. It has also been noted that this type of rearrangements also occur in regulating gene expression during normal developmental processes. This type of gene regulation mechanism occurs in both prokaryotic and eukaryotic cells.
In yeast, there are two mating type genes a and a a type cells readily mate with cells of the a type while cells of the same type are incapable of mating, However, yeast can overcome this barrier by replacing a a gene of the opposite mating type at the mating type locus.
This is possible cis the master inactive copies of the a and a a genes are present in the DNA sequence. This replacement can be possible in vitro by synthesizing master active genes and then inserted in the mating type locus (Fig. 16.23).
The newly inserted gene is then capable of transcription. This type of method of regulation is known as Cassette mechanism as the insertion of new gene sequences into the same location indicates which genes will be expressed—like putting cassettes into a tape recorder for hearing a specific music.
Again, DNA rearrangement process is active in production of multiple kinds of antibodies by joining different variable regions with the same constant region.
(e) Changes in DNA Conformation:
Gene activity can also be regulated by altering the three-dimensional conformation of DNA structure. One type of this change is altering the coiling of the DNA double helix. During transcription, the unwinding of DNA helix in a localised region occurs in order to allow base pairing for the synthesis of new RNA chain.
It has been established that DNA of both prokaryotic and eukaryotic cells is formed loops whose ends are fixed as DNA cannot rotate. This type of localised unwinding in a fixed structure will form a tension in the DNA helix producing a positive supercoil.
This condition is not favourable for transcription, it thus hampers the rate of transcription. This problem in bacteria is overcome by the enzyme DNA gyrase which takes the energy from the breakdown of ATP and produces negative supercoil (Fig. 16.24).
When transcription takes place through localised unwinding of the DNA helix in this negative supercoiled structure, the tension developed due to the unwinding cannot generate any positive supercoils and thus decrease in the rate of transcription can be avoided. The role of DNA gyrase in regulating gene activity has been confirmed using inhibitors of DNA gyrase.
Again, changes in the DNA conformation has been observed with the discovery of Z-DNA by Rich in eukaryotic cells. The normal form of DNA, known as B-DNA, has the arrangement of sugar phosphate backbone as a smooth right-handed helix. But Z-DNA is a left-handed helix with a zigzag sugar phosphate chain (Fig. 16.25).
The presence of Z-DNA has been observed in enhancers and other control sequences of viral DNA molecules resulting in the establishment of an idea that it has a role in regulating gene activity. Although Rich first observed Z-DNA in synthetic molecules, antibodies against Z- DNA have been found in normal cells. One possibility factor in the formation of Z-DNA in the normal cell may be the methylation of CG- sequences.
8. Transcription Signals for Gene Control in Eukaryotic Cells:
Large number of gene-activating ,signals are produced within the cells-tissues during development which help in the initiation of gene expression. Of these the most common signals are the different types of hormones in animals.
Some hormones may directly control the activities of genes or affect the action of enzymes already present in the cell. These hormones may be produced in any distant cell and then are transported to the target cell/organ through the circulatory system.
Thyroid hormones and glucocorticoids (steroids) affect many genes in tissues rather than single gene. Insulin and growth hormones have similar function in many tissues. There are also some protein factors with specific functions.
For example, low molecular proteins—say Interferon’s—help cells to become resistant to the growth of different types of viruses. Interferon has a special cell- protein interaction for studying gene regulation in many cells.
Besides steroids and hormones, there are some polypeptides or proteins which exert their effect in being attached to cell surfaces. After binding to the cell surface, some proteins are transported inside where they play an active role in signalling genes. Again, cell- to-cell contacts have also a role in the control of eukaryotic gene expression.
In lower eukaryotes like yeast, environmental signals and nutritional stress have a definite role in controlling gene expression. For example, starvation of amino acids in the culture of yeast cells lead to the increase in the levels of bio-synthetic enzymes to increase the production of these amino acids like tryptophan, histidine, isoleucine and valine etc.
Some effect of nutritional stresses on Gene actions of mammalian cells has been noted. Most of the mammalian cells have the ability to synthesize purines and pyrimidine’s so the media do not require these compounds during culture of these cells. But when adenine (purine) is added to the medium, cells prepare 100% of the adenine and guanine from the adenine added to the medium.
Now if uridine is added to the medium, cells do not stop the synthesis of uracil and cytosine residues. That means adenine is a signal that inhibits at any stop of the purine synthesis pathway while pyrimidine (uridine) does not show any inhibition effect in the pyrimidine synthesis pathway (Fig. 16.26).
Some eukaryotic cells again show response in gene activation to an increase in temperature or heat shock with the synthesis of some special type of proteins.
Fig. 16.26: Regulation of Purine and Pyrimidine Biosynthesis: