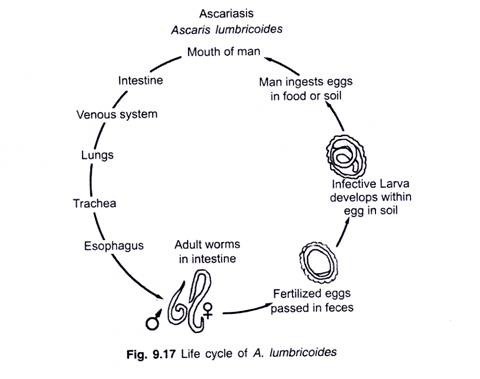
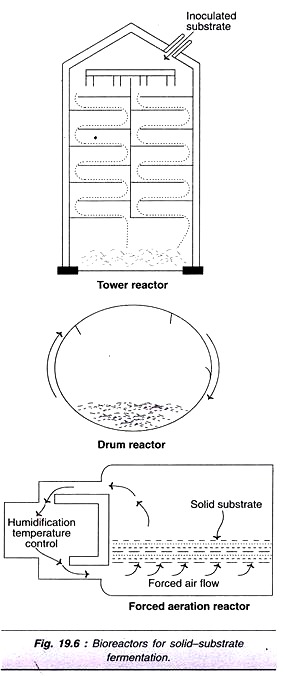
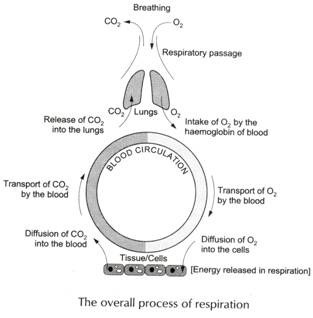
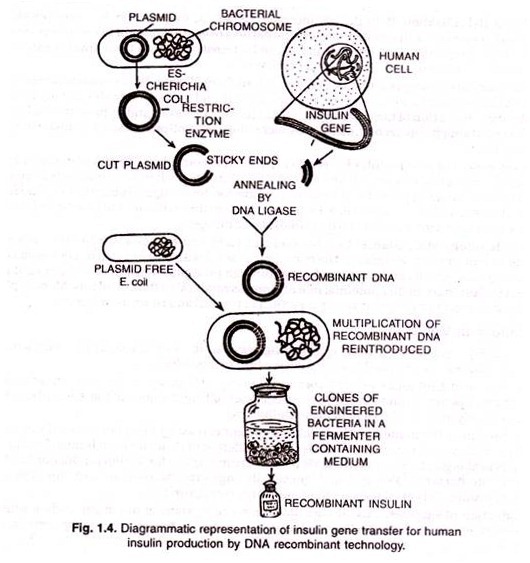
ADVERTISEMENTS:
Let us make an in-depth study of the types and chemical properties of lipids. The three types of lipids are: (A) Simple Lipids (B) Compound Lipids (C) Derived Lipids and chemical properties of lipids are: 1. Saponification 2. Saponification Number 3. Iodine Number and 4. Rancidity.
Types of Lipids:
(A) Simple Lipids:
They are esters of fatty acids with alcohol. Depending upon the alcohol, they are further classified as:
1. Neutral Fats:
ADVERTISEMENTS:
These are esters of fatty acids with glycerol (a trihydric alcohol). These are known as triacylglycerol’s (TAG) or triglycerides.
R1, R2, and R3 are the three fatty acids. All the three may be the same or different. If all the three Rs are the same, then it may be, Tripalmitin-3 palmitic acids esterified with glycerol. Tristearin-3 stearic acids esterified with glycerol. If the ‘R’ groups are different then it is spelled out as Palmito-stearo-olein indicating that glycerol is esterified with palmitic acid, stearic acid and oleic acid.
2. Waxes:
ADVERTISEMENTS:
These are esters of long chain fatty acids with long chain alcohol, e.g., Bee wax is ester of oleic acid (18 carbons) and oleyl alcohol (18 carbons).
(B) Compound Lipids:
Simple lipids in combination with some other group are called compound lipids.
Depending upon the group attached (prosthetic group) the compound lipids are further classified as:
1. Phospholipids:
They contain a phosphoric acid as the prosthetic group.
Depending upon the alcohol present they are further classified as:
(a) Glycerophospholipids:
They contain the alcohol-glycerol. The components of glycerophospholipids are glycerol, two fatty acids (the one at α-position is saturated fatty acid and the other at β-position is unsaturated), phosphoric acid and a base. Glycerol, fatty acids and phosphate together form a phosphatide to which a base is attached. Depending upon the base present there are various glycerophospholipids.
Phophatidyl choline or lecithin:
ADVERTISEMENTS:
Here the base is choline
Phosphatidyl ethanolamine or cephalin:
Here the base is ethanol amine, attached through — OH group.
Phosphatidyl serine:
Here the base is the amino acid serine.
Phosphatidyl inositol:
ADVERTISEMENTS:
Here the base is inositol.
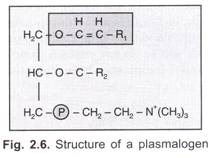
Plasminogen:
Here one of the fatty acids of the phosphatide is replaced by a long chain aldehyde which is in an enolic form. The base may be choline or ethanolamine.
Cardiolipin or di-phosphatidyl glycerol:
Here two phosphatide groups are linked together through a glycerol.
(b) Sphingophospholipids:
These phospholipids have sphingol as the alcohol. Sphingol is an amino alcohol with a chain length of 18 carbons having a double bond at trans delta 4 position. An example of sphingophospholipid or sphingolipid is sphingomyelin, which contains a fatty acid at the amino group (and this combination, i.e., sphingol and fatty acid is known as ceramide), a phosphoric acid at the primary alcohol and the base choline is attached to this phosphate group.
2. Glycolipids:
ADVERTISEMENTS:
These lipids contain a carbohydrate attached to the sphingol at the primary alcohol. They are also known as glycosphingosides or cerebrosides.
(a) Glucocerebrosides:
If the sugar is glucose, then they are called as glucocerebrosides.
(b) Galactocerebrosides:
If the sugar is galactose then they are called as galactocerebrosides.
ADVERTISEMENTS:
(c) Gangliosides:
These are complex sphingolipids made up of several sugar units, viz., glucose, galactose, galactosamine and N-acetyl-neuramic acid or sialic acid.
3. Lipoproteins:
These are lipids in conjugation with proteins. They mainly function for the transport of lipids (hydrophobic) through the blood (hydrophilic).
The different types of lipoproteins and their composition is:
These lipoproteins are classified depending upon their densities in water. The density of a lipoprotein depends upon the fat content of that lipoprotein, more the fat content lower the density and hence it floats on the surface of water (vice versa). The protein part in the lipoprotein is known as apoprotein. The various types of apoproteins found in lipoproteins are apoprotein- A, B, C, D, E. Lipoproteins also constitute the combination of membrane proteins with membrane lipids.
(C) Derived Lipids:
These are the compounds obtained on hydrolysis of simple and compound lipids. They also constitute all those compounds that are related to fatty acids. They include fatty acids, steroids, eicosanoids (prostanoids- prostaglandins, prostacyclins, thromboxanes-leukotrienes and lipoxins), carotenoids, etc.
1. Steroids:
All compounds containing the cyclo-pentano-perhydro-phenanthrene ring are called steroids. The most abundant steroids in the human body are the sterols, i.e., an alcohol (—OH) group is attached to the steroid nucleus, e.g., cholesterol, ergosterol, bile acids, sex hormones, adrenal cortical hormones and vitamin D3.
Cholesterol is the major sterol in the body. It is a constituent of cell membrane and provides rigidity to it. Cholesterol acts as the precursor for all the other steroids in the body, viz., testosterone, estrogen, progesterone, vitamin-D, bile salts, corticosteroids, etc.
2. Prostaglandins:
They are the derivatives of polyunsaturated fatty acids, mainly the arachidonic acid (C20) or even linoleic acid (C18). They are 20 carbon fatty acids with a 5 membered ring.
There are four types of prostaglandins PGE, PGF, PGA and PGB. But only PGE and PGF are important. The E group of prostaglandins contains a keto group at C-9, two -OH groups at C-11 and C-15 positions. The various types of PGE are E1, E2, E3.
The PGF groups contain -OH group at all the three positions. The various types of PGF are F1, F2 and F3. Among the subtypes there are two more sub-subtypes in each of the prostaglandins, i.e., α and β.
Functions of Prostaglandins:
Prostaglandins are synthesized in all tissues and cells except RBC. They act as local hormones and are destroyed immediately.
Other functions include as under:
1. They act as vasopressors and hence lower the blood pressure.
2. They are used in induction of labour, termination of pregnancy and prevention of conception.
3. They facilitate fertilization of the ovum.
4. They are used in the treatment of gastric ulcers, as they diminish HCl secretion.
5. They are used to prevent inflammation.
6. They are used in asthma and congenital heart diseases.
7. They promote platelet aggregation (after conversion to thromboxane’s).
8. PGE2 acts as a messenger between hormone receptor and adenylate cyclase enzyme.
Chemical Properties of Lipids:
1. Saponification:
Hydrolysis of TAG with KOH or NaOH is called saponification or soap formation. These soaps are the household soaps. Sodium soaps are hard and potassium soaps are soft. Detergents have acidic group like sulphuric acid attached to the fatty acids.
2. Saponification Number:
It is the number of milligrams of KOH required to saponify the free and combined fat in 1 gram of a given fat. A high saponification number indicates that the fat is made up of low molecular weight fatty acids and vice versa.
3. Iodine Number:
It is the grams of iodine required to saturate 100 grams of fat. It is an indication of unsaturation.
4. Rancidity:
Fats contaminated with enzymes like lipase undergo partial hydrolysis and oxidation of unsaturated fatty acids at the double bonds. This is even brought about by the atmospheric moisture and temperature. Due to this, there is release of hydrogen peroxide giving a bad odour and taste to the fat. This fat is said to be rancid and the process is known as rancidity. Rancidity can be prevented by antioxidants like vitamin E, vitamin C, phenols, hydroquinone’s, etc.
Molecular structure of a cell membrane:
Each cell and sub-cellular organelles are surrounded by a lipid bi-layer. 70 to 80 per cent are polar lipids and the remainder is mostly protein. The lipid part of the membrane is polar or amphipathic lipid largely phosphoglycerides, some amounts of sphingolipids and a negligible amount of triacylglycerol’s. Cholesterol and cholesterol esters are also present in the membrane.
Membranes are very thin from 6 to 9 nm, flexible and fluid. They are freely permeable to water but impermeable to electrically charged ions like Na+, CI– or H+ and to polar but uncharged molecules like sugars. These impermeable substances are transported with the help of membrane transport proteins. On the other hand lipid soluble molecules readily pass through membranes.