ADVERTISEMENTS:
The following points highlight the top nine instruments that are used in microbiology. The instruments are: 1. Spectrophotometry 2.Autoradiography 3. Centrifugation 4. Electrophoresis 5. Gel Filtration 6. Hot-Air Oven 7. Autoclave 8. Laminar Air Flow 9. Incubator.
Instrument # 1. Spectrophotometry:
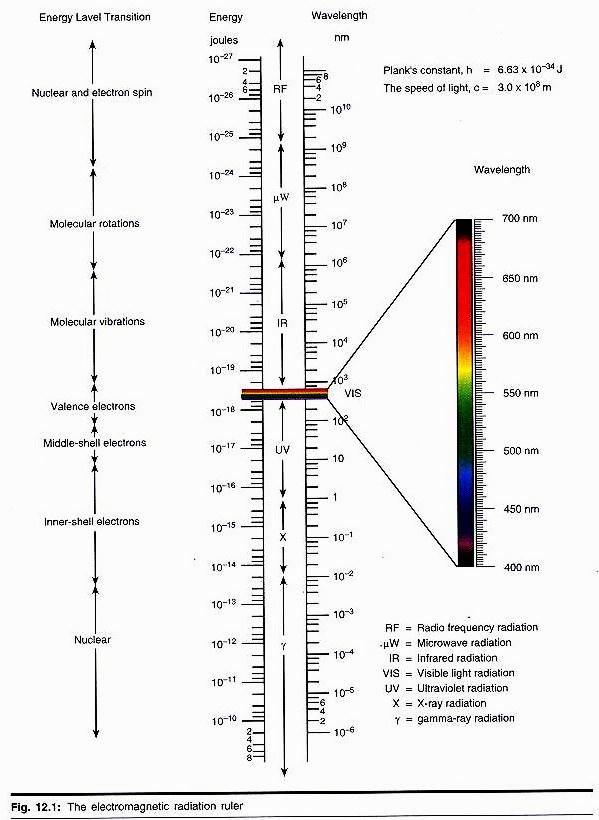
A spectrophotometer is a sophisticated type of colorimeter where monochromatic light is provided by a grating or prism. The band width of the light passed by a filter is quite broad, so that it may be difficult to distinguish between two compounds of closely related absorption with a colorimeter.
A spectrophotometer is then required, when the two peaks can be selected on the monochromator. Some compounds are absorbed in the UV region and their concentration is not possible to determine by using colorimeter. For such purposes, concentration is determined by spectrophotometer which also operates below 190 nm.
i. Absorption Spectra:
Many compounds have characteristic absorption spectra in the UV and visible region so that identification of these substances in a mixture is possible.
Glass cuvettes are much cheaper than silica (quartz) and are used wherever possible. Their main limitation is that glass absorbs UV radiation and quartz can be used below 360 nm, so quartz cuvette are employed below this wavelength. The glass cuvette range varies from 360-800 nm, while quartz cuvette ranges from 200-800 nm. A tungston lamp produces a broad range of radiant energy down to about 360 nm.
To obtain the UV region of the spectrum a deuterium lamp is used in the range 360-400 nm, then a blue filter is placed in the light beam read against a reagent blank which contains everything except the compound to be measured.
The blank is first placed in the instrument and the scale adjusted to zero extinction (100% transmittance) before reading any solution. Alternatively, the extinction can be read against distilled water and the absorbance of the blank substracted from that of the test solution.
ii. Absorption Spectrum and Extinction Coefficient:
ADVERTISEMENTS:
The Bonguer-Lambert-Beer law stated that
E = c × d where c = concentration and d = light path; E-molar extinction coefficient.
It stated that the extinction (E) is proportional to the light path and to concentration c of the absorbing substance. The proportionality constant is the extinction of the substance at a concentration of unity with a light path of 1 cm.
With the unit mole/litre (M) for the concentration c, the molar extinction coefficient has the dimensions M-1 × cm-1. The extinction coefficient based on 1 mole/cm3 is 1000 times the molar extinction coefficient based on 1 mole/litre; for example NADH
340 nm = 6.22 × 103m-1 × cm-1
iii. Absorption Spectra of Different Compounds:
Many compounds have characteristic absorption spectra in the UV and visible region so that identification of these materials in a mixture is easily possible (Fig. 35.8).
The most frequently used wavelength in the UV region is 340 nm. At this wavelength, the reduced forms of the pyridine nucleotide coenzyme NADH2 and NADPH2 absorb strongly, while the oxidised forms do not (Fig. 35.9). NAD+ has a typical dinucleotide structure.
(i) Proteins:
ADVERTISEMENTS:
Proteins absorb strongly at 280 nm according to their content of the amino acids tyrosine and tryptophan and this provides a sensitive and non-destructive form assay. Proteins also absorb in the far ultraviolet because of the peptide bond.
(ii) Nucleic Acid:
Nucleic acids and their component bases show maximum absorption in the region of 260 nm. The extent of the absorption of nucleic acids is a measure of their integrity, since the partially degraded acids absorb more strongly than the native materials. The spectra of the component bases are also sufficiently different to be used in their identification.
ADVERTISEMENTS:
(iii) Haemoglobins:
When haemoglobins are modified by the effect of certain drugs or carbon monoxide, characteristic shifts of their absorption maximum occur, so that the presence of these modified forms can be detected and measured.
(iv) p-Triphenylphosphate:
This is a substrate used for phosphates, which catalyses the hydrolysis of the compound to p-nitro phenol and inorganic phosphate. In alkaline solution, the product p-nitrophenol gives a typical yellow colour with a maximum absorption at 405 nm. The substrate does not absorb in this region so the progress of the reaction in alkaline solution can be followed.
iv. Some Practical Points:
ADVERTISEMENTS:
The detailed operation of a particular instrument must, of course, be obtained by careful reading the instruction manual, but a few general points concerning the use and care of colorimeters and spectrophotometers are given below.
(i) Cleaning Cuvettes:
Cuvettes are cleaned by soaking in 50% v/v nitric acid and then thoroughly rinsed in distilled water.
(ii) Using the Cuvettes:
ADVERTISEMENTS:
First of all, fill the cuvettes with distilled water and check them against each other to correct for any small differences in optical properties. Always wipe the outside of the cuvettes with soft tissue paper before placing in the cell holder and do not handle them by the optical faces. When all the measurements have been taken, wash them with distilled water and leave in the inverted position to drain.
(iii) Absorption of Radiation by Cuvettes:
Glass cuvettes are much cheaper than silica ones and used wherever possible. Their main limitations is that glass absorbs ultraviolet radiation and they can be used at or above 360 nm, so silica cells are employed below this wavelength.
Glass cuvettes range: 360 – 800 nm
Silica (quartz) cuvettes range: 200 – 800 nm.
(iv) Light Source:
ADVERTISEMENTS:
A tungston lamp produces a broad range of radiant energy down to about 360 nm. To obtain the ultraviolet region of the spectrum a deuterium lamp is used as the light source. If the tungston lamp is used in the range 360-400 nm, then a blue filter is placed in the light beam.
(v) Photocells:
When working at wavelengths up to 625 nm a ‘blue’ photocell receives the emitted light and a ‘red’ photocell receives above this wavelength. Photocells are exposed to light for the shortest time necessary to take a reading in order to avoid fatigue.
(vi) Blanks:
The extinction of a solution is read against a reagent blank which contains everything except the compound to be measured. This blank is first placed in the instrument for the reading any test solutions. Alternatively, the extinction can be read against distilled water and absorbance of the blank subtracted from that of the test solution.
(vii) Replication:
ADVERTISEMENTS:
It is essential to prepare all blanks and standard solutions in duplicate so that an accurate standard curve can be constructed. In addition, the test solutions should also be prepared in duplicate wherever possible.
Instrument # 2. Autoradiography:
The use of radioactive radiations to obtain the photographic film of the test material, incorporated with the radioactive tracers, is called autoradiography and the film obtained is called autoradiograph. After development the irradiated areas appear on the film as dark areas corresponding to the distribution of the tracer.
i. Principle:
Autoradiography can be detected either directly with a scintillation counter or indirectly via their effect on photographic film. At the light microscopic level the autoradiography is based on the principle that if a photographic emulsion is brought into contact with radioactive material, the ionic radiations will convert the emulsion as dark spots of silver at certain points.
Radioactive substances are introduced into the test material either in a given chemical form or tagged with certain metabolic precursors. For example, nucleic acid can be made radioactive by incorporation of radioactive phosphate during nucleic acid synthesis. The newly synthesised nucleic acid thus becomes radioactively labelled.
ii. Types of Radiation:
The following three types of radiations are used in autoradiography:
(i) Alpha Rays:
The alpha rays particles which consist of 2 neutrons and 2 protons and are infact charged helium atoms. Radium 226 is their source.
(ii) Beta Rays:
The beta rays are electrons ejected or emitted by nuclei. The energy levels of these electrons may vary. When highly charged they are called hard beta as of 32p but soft beta is emitted from 14C.
(iii) Gamma Rays:
The gamma rays are electromagnetic rays and resemble X-rays. 60CO is gamma emitter.
iii. Procedure for Continuous Labelling:
(i) Exposure of Cells to 3HTDR (Tritiated Thymidine):
Tritiated thymidine (specific activity 12,600 in Ci/m mol) obtained from BARC should be added to the experimental material at the last ‘S’ phase prior to harvesting. Before preparation the material should be thoroughly washed for removing the traces of radioactive thymidine left in the medium which may cause excessive background radiation.
3HTDR + S phase of the organism → Labelled
(ii) Preparation of Film:
Air dried slides are to be stained with desired stain and then stripped film method may be followed:
Stripping film (AR-10 Kodak Ltd.) mounted on glass plates are to be removed from the refrigerator and allowed to cool down to room temperature. All the subsequent procedures are to be carried out in a dark room fitted with red light and low temperature (about 20°C).
Slides are marked on the specimen side and immersed in clean distilled water in coplin jar. The photographic plates are unwrapped, held with the emulsion side up and squares (4 × 4 cm2) were cut with a sharp scalpel. Using a pair of small forceps the squares are stripped off the plate and floated with emulsion side down in a tray containing distilled water.
When the squares straighten out, the slides are immersed in water with specimen side up and into a position beneath the square of the film. The slide is then lifted out of water.
The film covering the specimen straightens out and the overlapping ends flooded onto the other side of slide. Slides are now allowed to dry in a tray, packed in a slide box protected from light and kept with a small amount of silica gel in cold for 21 days exposure before developing.
Developing Autoradiographic Film:
All steps are carried out in the dark room fitted with lamp. The stored slides are painted on the reverse with euparol or nail polish to prevent shifting of the film and then kept for 5 minutes in developing solution (Kodak), washed thoroughly in distilled water fixed in Kodak metafix for 2 minutes. Again rinse in distilled water and dried, the film can now be observed to locate the deposition of silver grains.
Instrument # 3. Centrifugation:
i. Sand:
If the sand particles are suspended in water, the particles due to varying in their size, density and the velocity of the medium, settle down at different rates. On the other hand, the gravitational pull is also involved which is about 980 cm/sec2 or 1 g unit under normal condition. If the gravitational force is increased then particles called ‘light’ will also sediment.
Actually, if we apply the centrifugal force, the gravitational force is induced. As you know that the centrifugal force acts in a direction away from the centre of the axis. The faster the speed of rotation, the greater will be the force.
This can be expressed as below:
Centrifugal force = (angular velocity)2 × radius.
Angular velocity is related to rotations/min (rpm) by the following formula:
Angular velocity = 2π × rpm/60 resolution/sec.
The centrifugal force is generally expressed as relative centrifugal field (RCF) in g units as:
RCF = [4π2 (rpm)2]/3600 × 980 g units
or 1.11 × 10-5 rpm rg units
ii. Centrifuge:
This instrument is based on centrifugal forces. Basically, it has containers rotated around the central axis with the help of electric motor. Now-a-days, cooling centrifuges, high speed centrifuges and ultracentrifuges are available with the different types of rotors i.e. angle head and swinging bucket types.
In the angle type rotor, the sample kept at an angle of about 30° to the horizontal whereas, in the latter, the sample while spinning is horizontal. The most common centrifuge is clinical centrifuges. In the case of ultracentrifuges, the spun rotors produce force under vacuum to reduce friction.
iii. Zonal Centrifugation:
For the separation of the components of a mixture, the sample in solution is centrifuged. The sedimentation takes place in a solution present in a column. The density of the solution increases as we move down the column. The solution should be inert as a result of which gradient is formed.
This sample mixture is now put in a column which allows the sample to form different bands according to their rate of sedimentation. The components with high sedimentation rate are at lower end of the column.
The size and shape of the molecule also affect the sedimentation, as sedimentation coefficient is a function of a mass of the particle. The bands are formed at various positions which can be separated by puncturing the tube; bands are collected separately as depicted in the figure 35.12.
iv. Density Gradient Centrifugation:
The sample is mixed in a dense solution possessing low concentration and having fast diffusing property. The mixture is spun in centrifuge but before starting the process the sample is to be kept in a uniform mixture.
After centrifugation, the solution forms a density gradient and the sample components occupy those positions in the density gradient which correspond to their density. The bands of sample formed are separated by puncturing the tube. The method is also called isopycnic centrifugation (Fig. 35.13).
v. Differential Centrifugation:
In this type of centrifugation the components of a mixture are separated according to their size, shape and density. Generally bigger molecules reach the lower part of the coloumn before smaller one get sedimented. These particles which have the same size get sedimented according to their density difference whereas those particles which have same density are separated by differential centrifugation techniques.
In this method, a homogeneous solution of mixture of components is taken and centrifuged for a fixed duration (time) and the centrifugal field is also fixed. After a certain period of time, it is found that some components are sedimented and they form supernatant solution. The pellet part is taken out and again centrifuged for fixed duration at a particular centrifugal field. Because first centrifugation does not give a pure pellet.
Since homogenous solution was taken, hence few medium sized particles are present along with large size particles. By repeated centrifugation of the pellet portion only a pure pellet can be obtained. The supernatant fluid is separated from which the particles having lowest sedimentation rate can be separated out.
As the supernatant fluid left at last, has lost all large, medium, small sized particles and it contains only the smallest sized particle pellets, after repeated centrifugation, only large sized particles and thus is now pure. In the end, bands of sample components are formed the large sized particles at the bottom of the tube and the small sized particles present at the top of the tube. Thus, the different components of a mixture are separated.
Instrument # 4. Electrophoresis:
Tiseleus (1937) developed the process of electrophoresis. This is a process of separation of particles where on the basis of difference in charges and molecular size and shape the particles under the influence of an electric field migrate. Many bio-chemicals such as amino acids, peptides, proteins and nucleic acid possess ionizable groups.
The process takes place by dissolving the sample into a buffer solution and the supporting medium (such as starch for protein separation) used for casting the gel, also saturated with the buffer. The isoelectric point of the molecule and the buffer decided the charge on the molecule. If the buffer is at a above pH with that of isoelectric point, the sample will be negatively charged and will migrate towards the anode and vice-versa.
This method exploits the underlying fact.
On the application of electric field the electric force experienced by any particle can be written as:
Felec = qE
where q = Charge on the particle
E = Applied field
The maximum velocity against the fractional force of the medium.
F fract. = Vf
where f fract. = Fractional force
V = Velocity
f = coefficient of fraction
If the applied field is constant then,
qE = fV
µ = V-2/E = q/f
Here µ is defined as the velocity of the particle at an applied field.
Electrophoresis can be performed by both vertical as well as horizontal methods (Fig. 35.14 and Fig. 35.15). Both horizontal and vertical systems of electrophoresis chiefly comprise of two parts, the electric supply unit and the electrophoretic unit. The difference lies in the mode of arrangement.
Method:
In gel electrophoresis, the agarose gel is present between two glass plates placed together by means of plastic chips. It is immersed in a buffer solution which maintains the required pH for the molecule to remain charged and thus facilitates electrophoresis. The sample is applied by means of pipetting in micro-quantity.
The comb is removed after the sample application. The comb adds the sample in the upper buffer chamber. If the substance to be separated is a protein, the pH is maintained at pH 9 and the reason is that protein molecules are negatively charged at this pH. To ensure further uniform distribution of charged molecules agents like SDS (sodium dodecyl sulphate) is added.
After application of electric field or lower side, they are absorbed into various bands depending upon this charge and molecular weight, the small sized particles move faster and are close to the anode, the developed electrophoresis plate is dried and analysed by using various techniques.
The electrophoresis is of several types, such as paper, slab gel, disc electrophoresis, etc. This technique is specially useful in medical microbiology where in the bacterial antigens (proteins) are electrophorized and the composition helps in preparing an antibody against it.
Instrument # 5. Gel Filteration:
Biological macromolecules form a class of substances with special functions which are controlled in vivo by small changes in the environment.
Changes in the pH, concentration of metal ions, cofactors etc. may have a profound effect on the molecules being studied and it is clearly necessary to have available mild separation techniques which operate independently of these factors.
Gel filteration is one of these techniques. A gel filteration separation can be performed in the presence of essential ions or cofactors, detergents, urea, at high or low ionic strength, at 37°C or in the cold room.
As a solute passes down a chromatographic bed its movement depends upon the bulk flow of the mobile phase and upon the Brownian motion if the solute molecules which causes their diffusion both into and out of the stationary phase. The separation in gel filtration depends on the different abilities of the various sample molecules which never enter the stationary phase, move through the chromatographic bed fastest.
Smaller molecules, which can enter the gel pores, move more slowly through the column, since they spend a proportion of their time in the stationary phase.
The choice of a suitable gel for filtration is basically a case of finding the gel whose fractionation range covers the range of molecular sizes in the sample to be fractionated, since molecules of biochemical interest may range in size from a few hundred to many millions in molecular weight.
The most common media used include sephadex G-types (sephadex is a bead- forming cross-linked dextran gel which swells in water and aqueous salt solution). It is stable in the pH range 2-12 and in buffers containing dissociating agents such as urea and detergents and can be sterilized by autoclaving. Besides, sephadex, sepharose is also used and it is a bead forming agarose gel stable in aqueous suspension pH range 4-9.
Sepharose melts on heating and should not be used above 40°C or autoclaved. Sepharose provides an excellent medium for the fractionation of high molecular weight substances such as protein complexes and polysaccharides. Besides, sepharose has been proved to be a excellent medium for immobilisation of enzymes, antibodies hormones and other ligands for affinity chromatography.
A latest introduction of media for gel filteration is sephacryl (which is prepared for covalently cross-linking ally (dextran with N-N- methylene bisacrylamide) to yield a highly stable matrix. It can be used in an aqueous buffer system in concentration urea or guanidine hydrochloride and in a number of organic solvents. The covalently cross-linked matrix cannot melt on heating and sephacryl is conveniently sterilized by autoclaving.
Instrument # 6. Hot Air Oven:
This is a dry air type sterilizer with three walls and two air spaces. The outer walls are made up of thick asbestos to reduce the radiation of heat. The hot air steriliser is operated electrically. In this case the heater coil is either be placed at the bottom of the oven or on the side walls. A convection current travel a complete circuit through the wall space and interior of the oven. The temperature inside the oven is controlled by thermostat.
i. Principle:
The hot air steriliser is operated at a temperature of 160 to 180°C for a period of one and a half hour. If the temperature goes above 180°C there is a danger of cotton being charred.
The hot air steriliser is used for sterilizing all kinds of laboratory glassware, such as test tubes, Petri dishes, pipettes, flasks, bottles, etc. Other materials which will not be burnt at high temperature may also be sterilized in hot air steriliser. Petri dishes may also be put in metal cans or wrapped with paper and placed inside the steriliser.
ii. Precaution:
It is necessary to check the proper temperature at which the materials are sterilized. Under no circumstances should the hot air oven be used to sterilize culture media, as the liquids will boil to dryness. There should be temperature controlling device for maintaining the temperature required for sterilization.
iii. Uses:
The hot air sterilizer is used for sterilizing laboratory glass ware such as test tubes, Petri dishes, pipettes, flasks, bottles and other materials which will not be burned at higher temperature.
Instrument # 7. Autoclave:
The autoclave is a cylindrical vessel having double walls around all parts except the upper side. It is built to withstand the steam pressure of at least 30 lb per sq. inch.
i., Principle:
The principle used here is to increase the temperature of steam (gas) in a closed system that increases its temperature. The water molecules become more aggregated that increases their penetration considerably.
The water boils at 100°C depending upon the vapour pressure of the atmosphere. The temperature will be increased if the vapour pressure is increased. This relationship between pressure and temperature is shown in Table 35.1.
The autoclave is usually operated at 15 Ib/sq. inch steam pressure for 30 min., which as seen from the above table corresponds to 121.5°C. This temperature for a period of 30 min. is sufficient to kill all the spores and vegetative cells of microorganisms.
ii. Precautions:
The following precautions are to be taken: The level of water should be checked before operating. The air should be completely evacuated from the autoclave and the steam must have access to the materials to be sterilized.
For example, if a material such as cotton wool or glass beads is to be sterilized in a glass bottle closed with rubber stopper the sterilization would not be complete as steam cannot pass through the rubber stopper.
iii. Procedure:
Sufficient amount of water is placed inside the autoclave. The material is placed inside the autoclave for sterilization. The cotton plug should be covered with a piece of butter paper so that the plug does not wet. The lid of autoclave should be tightened with the help of screws, then switch on the plug.
The steam outlet is kept open till we feel that the air from inside the autoclave has been evacuated and then close the steam outlet. The pressure is allowed to remain at 15 Ib/sq2 inch for 15-30 min., is done by controlling the steam in the valve.
After 30 minutes switch off the current and let the autoclave cool down and thus the pressure comes down to zero mark. Then the autoclave is cooled down, the lid is opened and taken out the materials.
iv. Uses:
The autoclave is used to sterilize usual non-carbohydrate media, broths and agar media, contaminated media, aprons, rubber tubings, rubber gloves etc. This types of sterilization is also used in the commercial canning of fruits and vegetables and also in order to manufacture sterilized milk.
Instrument # 8. Laminar Air Flow:
Laminar air flow is an equipment having an air blower in the rear side of the chamber which can produce air flow with uniform velocity along parallel flow lines.
There is a special filter system- high efficiency particulate air filter (HEPA filter) which can remove particles as small as 0.3 µm. In front of the blower, there is also peculiar mechanism from which the air blown from the blower produces uniform air velocity along parallel flow lines.
These are horizontal and vertical types:
i. Principle:
Laminar flow can produce dust free air current with uniform velocity along parallel flow lines which help in transferring the culture media bacteria free. Air is passed through these special filters into the enclosure and the filters does not allow any kind of microbes to enter into the system. Due to uniform velocity and parallel flow of air current we can perform pouring, plating, slanting, streaking without any kind of contamination.
ii. Precautions:
Following precautions should be taken care of before handling the apparatus: We should put off our shoes before entering to operate the apparatus. We should wash our hands with soap and we should not talk inside the chamber while doing experiment, otherwise there will be a chance for contamination with certain bacteria or microorganisms through air of our mouth.
iii. Procedure:
Dust particles are removed from the surface of the laminar flow with the help of smooth cloth using alcohol. The UV light should be switch on for 30 minutes before performing the experiment and the front covering glass of laminar flow is opened and kept properly. The air blower is set at the desired degree so that the air inside the chamber is to be expelled because the air which is already inside the chamber may be contaminated.
One should sit properly in front of the chamber and wash the hands and stage of the chamber again with alcohol to reduce contamination. All the experiments i.e. pouring, plating, streaking etc. are to be done within the flame zone of the sprit lamp. The required materials are to be placed side by side on the stage of laminar flow.
iv. Uses:
Within the chamber of laminar flow, we can transfer any media for culturing bacteria or fungi or any microbe without any contamination. The parallel and smooth air flow blown out from inside the chamber of the laminar flow is dust free.
Instrument # 9. Incubator:
An incubator is an equipment that consists of copper/steel chamber, around which warm water or air circulates either by electricity or by means of small gas flame. The temperature of the incubator is kept constant by thermostat control.
i. Principle:
Incubator is operated to culture or for growing an organism in a suitable medium at proper temperature. In an incubator the variation of temperature should not be more than one degree Celsius (1°C). In large incubator the variation of temperature goes up to 2 or 3° C.
Small square type incubators are better than large incubators. If a lower temperature than that of the room temperature is needed, the water, before circulating around the upper chamber, is directed by the thermostat to pass through an ice chest or a small boiler according to the requirement of temperature.
ii. Precautions:
The door of the incubator should be opened only when necessary. If the tubes are to be incubated for a long time or at higher temperature, the medium may become too dry due to excessive evaporation.
In such case, cotton plug should be pushed inside the neck of the tube and a media rubber cap should be placed to cover the plug. If the Petri dishes are to be incubated for a long time these may be placed in moist chamber with a damp sterile cotton wool at the bottom.
iii. Uses:
The method of incubation of culture depends upon the temperature and the oxygen requirements of the organism. For this purpose the incubator is used to maintain different temperature required for growth of organism in a bacteriological laboratory.