ADVERTISEMENTS:
In this article we will discuss about:- 1. Structure of Chloramphenicol 2. Antibiotic Spectrum of Chloramphenicol 3. Mechanism of Action.
Structure of Chloramphenicol:
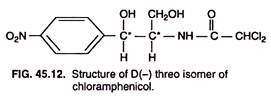
Chemically, chloramphenicol is a simple structure (Fig. 45.12) made up of nitrobenzene ring bonded with non-ionic chlorine. It consists of two unusual components—one nitro (-NO2) group and a dichloroacetyl (-COCHCl2) group. The molecule possesses two asymmetric carbon atoms (marked with asterisk in the figure). As a result, four optical isomers of chloramphenicol are possible. Of these isomers, only D (-) threo isomer is antibiotically active.
Antibiotic Spectrum of Chloramphenicol:
Chloramphenicol is bacteriostatic and a broad-spectrum antibiotic active against both gram-positive and gram-negative bacteria including rickettsia (cause of rocky-mountain spotted fever) and chlamydia. It is also found effective against Haemophilus influenzae causing meningitis.
ADVERTISEMENTS:
Chloramphenicol, as mentioned, has a broad-spectrum of activity but unfortunately is quite toxic and causes serious side effects. The most common side effect is a temporary or permanent depression of bone marrow function that results in cessation of formation of blood cells.
This antibiotic has been found to prevent incorporation of haemoglobin by the blood cells, leading to aplastic anaemia. It also causes thrombocytopenia and leucopenia (depletion of platelets and leucocytes). Other side effects are allergic responses or neurotoxic reactions. Thus, chloramphenicol is now used only in life-threatening situations when other suitable drugs are inadequate.
Mechanism of Action of Chloramphenicol:
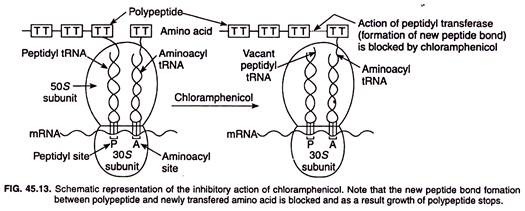
Chloramphenicol, like many other antibiotics such as streptomycin, gentamicin, tetracycline’s, erythromycin, etc. inhibits protein synthesis. It binds to the 23S rRNA on the 50S subunit of bacterial ribosome and inhibits the action of peptidyl transferase enzyme (Fig. 45.13).
ADVERTISEMENTS:
Peptidyl transferase transfers the growing polypeptide from the peptidyl tRNA to the amino acid bound to the aminoacyl tRNA leading to formation of a peptide bond and thereby lenthens the polypeptide by one amino acid rest.